













A gentle, comfortable1, shower-proof2 silicone foam dressing with breathable3,4 REACTIC◊ film technology.
ALLEVYN Gentle Border Dressings have a triple layer dressings that include a gentle silicone gel adhesive, a hydrocellular foam core, and a highly breathable REACTIC waterproof top film that lasts up to 7 days.
ALLEVYN Gentle Border Dressing maintain a moist wound environment1-3,5 that promotes healing.
Always read the label and follow the directions for use.
| Size | Dressings per pack |
| 7.5cm x 7.5cm | 2 dressings |
How do I use ALLEVYN Gentle Border Dressings?
1. Wash hands before and after treating the wound. Gently clean the wound and the surrounding skin with clean water and remove any visible dirt or debris.
2. Clean and dry the skin surrounding the wound and clip any excess hair close to the wound.
3. Ensure the absorbent pad area covers the entire wound or area to be protected before applying the dressing.
4. Open the pouch by peeling apart the flaps. Remove the dressing.
5. Start to remove the backing paper from the dressing.
6. Anchor the adhesive side of the dressing to the skin.
7. Smooth the dressing over the wound, removing the remaining backing paper.
8. Ensure the dressing is flat to the wound, free from creasing and has adhered all around the wound. The dressing may be repositioned as required.
ALLEVYN Gentle Border Dressings can be cut to fit smaller wounds or awkward areas. However, cutting the dressing compromises its bacterial barrier and waterproof properties. If the dressing has been cut, ensure any exposed foam areas are covered with an appropriate film dressing taking care not to cover the entire dressing.
• Care should be taken when using the dressing under medical devices near the nose and mouth, where moisture can build up and affect adherence, leading to dressing movement.
• Due to the gentle adhesive used, do not use this dressing to secure other medical devices to the patient.
• If reddening or sensitisation occurs discontinue use and consult a healthcare professional.
• Whilst showering, care must be taken to avoid wetting of the dressing. Do not expose to direct spray or submerge. Do not use with oxidizing agents such as hypochlorite solutions (e.g. EUSOL) or hydrogen peroxide as these can reduce the absorbency of the dressing.
ALLEVYN Gentle Border is a single use product. If used on more than one patient, cross-contamination or infection may occur. Opening the dressing pack compromises the sterile barrier therefore any unused dressing should not be retained for use at a later date. If used on infected wounds, the infection should be managed as per local clinical protocol.
Dressings are for healthcare professional use or for use by consumers.
Storage and handling
Store in a dry place.
Are ALLEVYN Gentle Border Dressings suitable for fragile or sensitive skin?
Yes, ALLEVYN Soft Silicone Foam Dressings are gentle on skin and are suitable for use on fragile or sensitive skin. Gentle silicone wound contact layer is non-adherent to the wound, minimally disruptive to healing tissue, low-allergenic, and provides secure adhesion.
What are ALLEVYN Gentle Border Dressings used for?
ALLEVYN Gentle Border Dressings may be used for minor weeping wounds such as:
• Cuts and lacerations
• Abrasions and grazes
• Blisters
• Minor post-operative wounds including stitches
• Skin tears
• Minor burns and scalds
Can ALLEVYN Gentle Border Dressings used for chronic wounds?
Under the direction of a healthcare professional, ALLEVYN Gentle Border Dressings may be used for wound management of serious wounds including:
• Leg ulcers
• Diabetic foot ulcers
• Surgical wounds
• First and second-degree burns
• Skin tears
1. ALLEVYN Gentle Border dressings can be used by lay persons under the supervision of Healthcare Professionals.
2. If you are concerned about your wound or have any questions about applying, monitoring or removing the dressing, contact your Healthcare Professional.
When should I replace ALLEVYN Gentle Border Dressings?
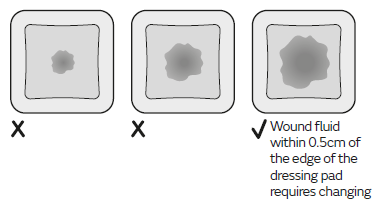
Fluid absorbed by the dressing will appear as a darker patch at the surface of the dressing. Inspect the dressing frequently to monitor the size of the darker patch. Dressings can be left in place for up to 7 days depending on the condition of the wound and the surrounding skin or until wound fluid (darker patch) is visible and approaches to within 0.5cm of the edge of the dressing pad, whichever is sooner.
 |
How do I remove ALLEVYN Gentle Border Dressings?
To remove the dressing, lift one corner and slowly peel back until completely removed from the wound. Throw away hygienically after use.
Can I use ALLEVYN Gentle Border Dressings with other treatments?
ALLEVYN Gentle Border Dressings can be used in conjunction with INTRASITE◊ GEL Hydrogel wound dressings under the direction of a healthcare professional.