Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today announces the first cases for revision knee replacement utilizing its CORI◊ Surgical System. Dr. Thorsten Seyler of Duke University performed the first cases on August 17, 2022, combining Smith+Nephew’s handheld robotics technology with its LEGION◊ Revision Knee System. Smith+Nephew is the first orthopaedics company to receive FDA 510(k) clearance for a revision indication using a robotics-assisted platform.
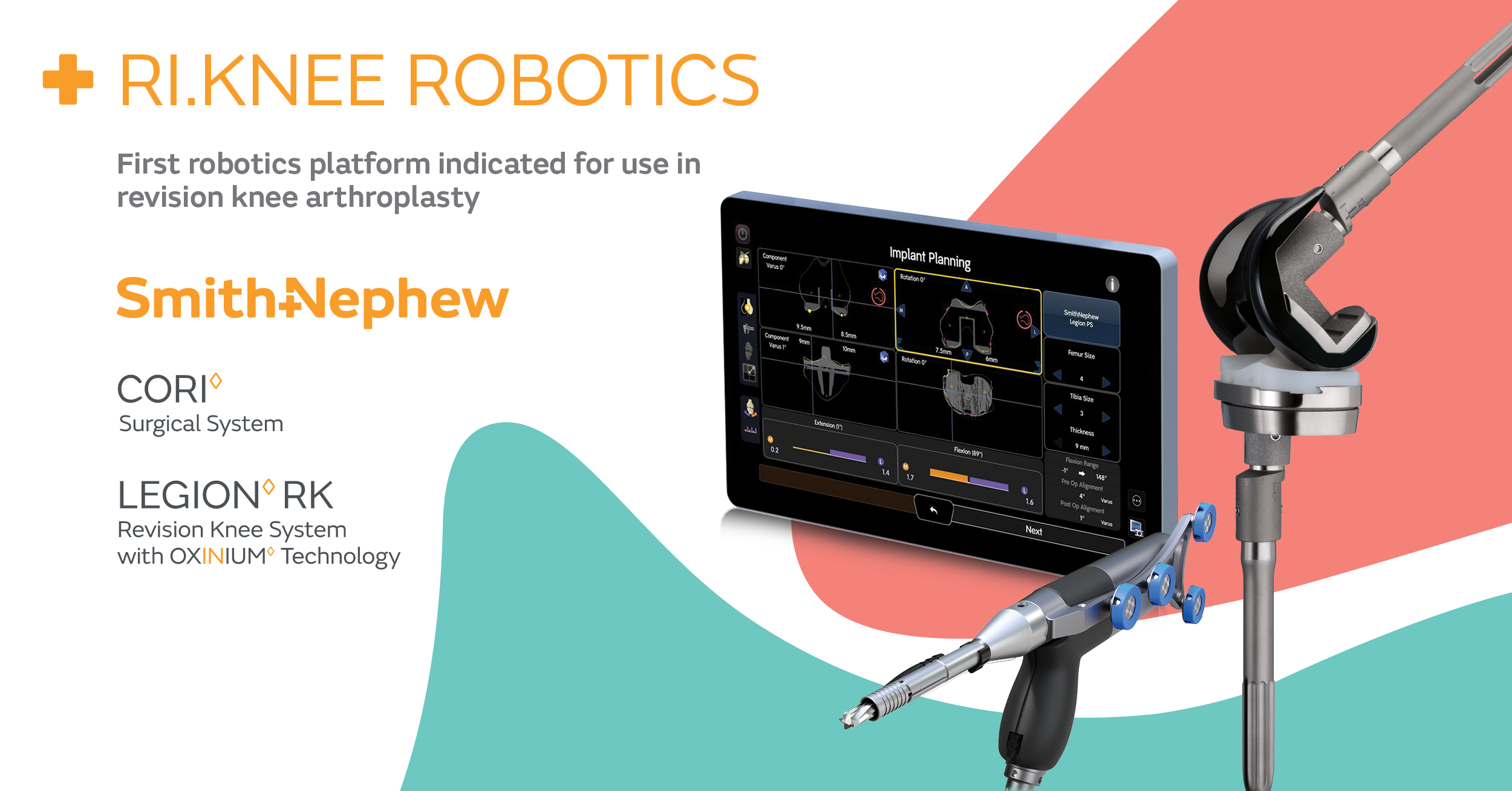
RI.KNEE ROBOTICS utilizes image-free smart mapping, eliminating the need for pre-operative CT/MRI scans and the potential for image distortion due to in situ components from the primary procedure. Instead, surgeons are able to build patient-specific 3D models of the joint, register anatomy and bony defects after implant extraction, intra-operatively gap balance in real-time, and accurately precision mill for final placement of components.1-4
“The ability to visualize and create symmetric and balanced flexion and extension gaps with the CORI handheld robotic system has made one of the most challenging tasks in revision TKA an easier undertaking. I have never used more posterior stabilized over constrained bearings in a revision scenario. Additionally, the image-free system also allows for accurate mapping of bone defects after implant removal and enables surgeons to use a bone preserving approach to revision TKA," said Dr. Seyler.
Revision knee surgery using robotics expands opportunity for health care professionals
Surgeons can now experience the power and versatility of one robotics platform when using the CORI Surgical System. With broader capabilities and expanded indications, it can address robotic-assisted total, partial, and now revision knee arthroplasty, along with computer-guided total hip arthroplasty. By coupling robotics with Smith+Nephew’s clinically-proven LEGION RK System with OXINIUM◊ Technology5*, surgeons can experience a truly comprehensive implant and technology portfolio.
“Being first to market with a revision indication for robotic-assisted knee replacement surgery is a significant milestone in orthopaedics,” said Randy Kilburn, Executive Vice President & General Manager, Orthopaedic Reconstruction, Robotics and Digital for Smith+Nephew. “Our ability to offer a robotic-assisted solution for partial, total, and now revision knee arthroplasty using a single platform is a true differentiator, especially when it potentially simplifies a complex procedure and maximizes the system's capabilities for surgeons to restore patient lives and live Life Unlimited.”
Over the last six months, Smith+Nephew has launched RI.HIP NAVIGATION, RI.HIP MODELER, “Cementless” CONCELOC◊ Advanced Porous Titanium 3D printing technology with LEGION◊ CONCELOC Cementless Total Knee System, and now a revision knee indication. All are supported by the CORI◊ Surgical System.
To learn more about the latest advancements in orthopaedic reconstruction and robotics, please click here.
Enquiries
|
Media |
|
|
David Snyder |
+1 978-749-1440 |
|
Smith+Nephew
|
|
*We are grateful to the Healthcare Quality Improvement Partnership (HQIP), the NJR Steering Committee and staff at the NJR Centre for facilitating this work. {Additional Contributors to be added where necessary}. The views expressed represent those of Smith+Nephew and do not necessarily reflect those of the National Joint Registry Steering Committee or the Health Quality Improvement Partnership (HQIP) who do not vouch for how the information is presented.
The data used for this analysis was obtained from the National Joint Registry ("NJR"), part of the Healthcare Quality Improvement Partnership (“HQIP”). HQIP, the NJR and/or its contractor, Northgate Public Services (UK) Limited ("NPS") take no responsibility (except as prohibited by law) for the accuracy, currency, reliability and correctness of any data used or referred to in this report, nor for the accuracy, currency, reliability and correctness of links or references to other information sources and disclaims all warranties in relation to such data, links and references to the maximum extent permitted by legislation including any duty of care to third party readers of the data analysis.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 18,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.2 billion in 2021. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as "aim", "plan", "intend", "anticipate", "well-placed", "believe", "estimate", "expect", "target", "consider" and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew's most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew's expectations.
◊ Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
Reference
- Bollars P, Boeckxstaens A, Mievis J, Janssen D. The Learning Curve and Alignment Assessment of an Image-Free Handheld Robot in TKA: The First Patient Series in Europe. Poster presented at: 19th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery2019; New York, USA.
- Kopjar B, Schwarzkopf R, Chow J, et al. NAVIO Robotic Assisted Surgical System for Total Knee Arthroplasty Using JOURNEY II Guided-Motion Total Knee System. Poster presented at: ISTA 2-5 October, 2019; Toronto, Canada.
- Geller JA, Rossington A, Mitra R, Jaramaz B, Khare R, Netravali NA. Rate of learning curve and alignment accuracy of an image-free handheld robot for total Knee Arthroplasty. European Knee Society Arthroplasty Conference;2019; Valencia, Spain.
- Kaper BP, Villa A. Accuracy and Precision of a Handheld Robotic-guided Distal Femoral Osteotomy in Robotic-assisted Total Knee Arthroplasty. European Knee Society Arthroplasty Conference;2019; Valencia, Spain
- National Joint Registry for England, Wales and Northern Ireland: LEGION Revision OXINIUM (with Revision Tibial) implant summary report. 17 August 2022.
- We thank the patients and staff of all the hospitals in England, Wales and Northern Ireland who have contributed data to the National Joint Registry.